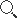Original Envitec OOM204 Medical oxygen sensor ,OOM202 O2 cell
Use the advantages: • Compliant with European MDD (CE certification) • Meets ISO 80601-2-55 • Designed and manufactured according to EN ISO 13485 • Accurate and reliable fast response • Resistant to N2 O • Excellent signal stability • High product quality • Short delivery times • Technical support • Made in Germany • FDA cleared
Technical Specifications
OOM204 Measurement range 0 % ... 100 % oxygen (at atmospheric pressure)
Nominal sensor lifetime ≥ 500 000 % volume oxygen hours
Output in ambient air 9 mV ... 13.5 mV (each of two channels)
Output difference 1.25 mV (between the two channels)
Electrical interface 3 pin (Molex® 22-11-1031)
Accuracy meets ISO 80601-2-55 requirements Repeatability < 1 % volume O2 at constant temperature and pressure
Linearity error < 3 % relative
Response time < 12 s to 90 % of final value
Zero offset voltage < 200 µV in 100 % nitrogen, applied for 5 min
Cross interference meets ISO 80601-2-55 requirements
Influence of humidity -0.03 % rel. per % RH at 25 °C
Pressure range 0.6 bar ... 2 bar (ppO2 0 ... 1250 mbar O2 )
Influence of pressure proportional to change in oxygen partial pressure
Influence of mechanical shock < 1 % relative after a fall from 1 m
Operating temperature 0 °C ... +50 °C
Temperature compensation built-in NTC compensation
Effect of temperature compensation (steady state) between +25 °C and +40 °C: 3 % relative error between 0 °C and +50 °C: 8 % relative error
Operating humidity 0 % ... 99 % RH non-condensing
Long term output drift < 1 % volume oxygen per month typically < -15 % relative over lifetime
Storage temperature -20 °C ... +50 °C
Recommended storage +5 °C … +15 °C
Recommended load ≥ 10 kOhms
Warm-up time < 30 minutes, after replacement of sensor
Weight approximately 28 grams
Part number 01-00-0097
Manufacturer date will be newest.
If you are interested above products,you can send inquiry as follow:
E-mail: jenny@upnmed.com
Whatsapp:+8613670105485
*Disclaimer: All the registered trademarks, product names, models, etc. shown in the above contents are owned by the original holder or the original manufacturer. This is only used to explain the compatibility of the UpnMed products, and nothing else! All the above information is for reference only, and should not be used as a working guide for medical institutions or related units. Otherwise, any consequences will be irrelevant to the company.